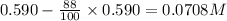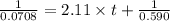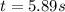Consider this reaction: →2NH3g+N2g3H2g At a certain temperature it obeys this rate law: rate =2.11·M−1s−1NH32 Suppose a vessel contains NH3 at a concentration of 0.590M. Calculate how long it takes for the concentration of NH3 to decrease by 88.0%. You may assume no other reaction is important.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1

Chemistry, 23.06.2019 08:00
Which of the following notations would be the appropriate and final way to display the formula for magnesium chloride a. mgcl2 b. mg+2cl–1 c. mgcl2 d. mgcl
Answers: 2
You know the right answer?
Consider this reaction: →2NH3g+N2g3H2g At a certain temperature it obeys this rate law: rate =2.11·M...
Questions


Mathematics, 04.02.2021 02:20

Mathematics, 04.02.2021 02:20

English, 04.02.2021 02:20

History, 04.02.2021 02:20

Mathematics, 04.02.2021 02:20



Mathematics, 04.02.2021 02:20

Mathematics, 04.02.2021 02:20




Mathematics, 04.02.2021 02:20

History, 04.02.2021 02:20

Mathematics, 04.02.2021 02:20




Mathematics, 04.02.2021 02:30

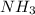 to decrease by 88.0%.
to decrease by 88.0%. 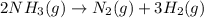
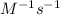 , the kinetics must be second order.
, the kinetics must be second order.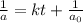
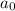 = initiaal concentration = 0.590 M
= initiaal concentration = 0.590 M