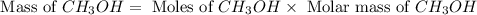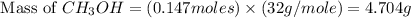Based on the ideal gas law, there is a simple equivalency that exists between the amount of gas and the volume it occupies. At standard temperature and pressure (STP; 273.15 K and 1 atm, respectively), one mole of gas occupies 22.4 L of volume. What mass of methanol (CH3OH) could you form if you reacted 6.59 L of a gas mixture (at STP) that contains an equal number of carbon monoxide (CO) and hydrogen gas (H2) molecules?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

You know the right answer?
Based on the ideal gas law, there is a simple equivalency that exists between the amount of gas and...
Questions

Engineering, 29.11.2021 08:10

Social Studies, 29.11.2021 08:10

History, 29.11.2021 08:10


Mathematics, 29.11.2021 08:10


Social Studies, 29.11.2021 08:20

English, 29.11.2021 08:20





English, 29.11.2021 08:20

Mathematics, 29.11.2021 08:20

History, 29.11.2021 08:20

Mathematics, 29.11.2021 08:20


Physics, 29.11.2021 08:20

Social Studies, 29.11.2021 08:20

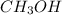 produced is, 4.704 grams.
produced is, 4.704 grams.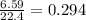 mole of gas mixture
mole of gas mixture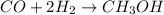
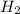 react with 1 mole of
react with 1 mole of 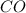
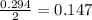 moles of
moles of