
Chemistry, 20.03.2020 01:29 latinotimo7643
The combustion of propane (C3H8) in the presence of excess oxygen yields CO2and H2O:C3H8(g) + 5O2(g) 3CO2(g) + 4H2O (g)When 2.5 mol of O2are consumed in thisreaction, mol of CO2are produced.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2
You know the right answer?
The combustion of propane (C3H8) in the presence of excess oxygen yields CO2and H2O:C3H8(g) + 5O2(g)...
Questions


Mathematics, 20.11.2020 01:30

Mathematics, 20.11.2020 01:30


History, 20.11.2020 01:30

History, 20.11.2020 01:30


Mathematics, 20.11.2020 01:30


Spanish, 20.11.2020 01:30

Computers and Technology, 20.11.2020 01:30


Mathematics, 20.11.2020 01:30


Mathematics, 20.11.2020 01:30

Mathematics, 20.11.2020 01:30

Physics, 20.11.2020 01:30

Mathematics, 20.11.2020 01:30


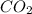 are produced.
are produced.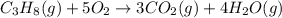
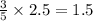 moles of carbon dioxide
moles of carbon dioxide

