
Chemistry, 20.03.2020 02:02 cookie42087
2.00 moles of CO2 and 1.50 moles of H2 are placed into a rigid 5.00-L container and they react according to the equation CO2(g) + H2(g) ⇄ CO(g) + H2O(g) K = 2.50 What will be the concentration of carbon monoxide when equilibrium is reached? 0.191 M 0.091 M 0.209 M (Your correct answer) 0.913 M 1.05 M

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
2.00 moles of CO2 and 1.50 moles of H2 are placed into a rigid 5.00-L container and they react accor...
Questions

Mathematics, 21.07.2021 21:10


English, 21.07.2021 21:10


Mathematics, 21.07.2021 21:10

Arts, 21.07.2021 21:10

Mathematics, 21.07.2021 21:10






Mathematics, 21.07.2021 21:10


Mathematics, 21.07.2021 21:10

Mathematics, 21.07.2021 21:10


Advanced Placement (AP), 21.07.2021 21:10


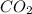 = 2.00 mole
= 2.00 mole = 1.50 mole
= 1.50 mole

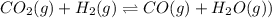
![K_c=\frac{[CO]\times [H_2O]}{[H_2]\times [CO_2]}](/tpl/images/0555/1888/1dc80.png)
![2.50=\frac{[x]\times [x]}{[0.300-x]\times [0.400-x]}](/tpl/images/0555/1888/54f3c.png)
 at equilibrium = x M = 0.209 M
at equilibrium = x M = 0.209 M

