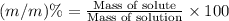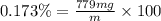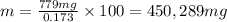Chemistry, 20.03.2020 02:35 StephenCurry34
You can practice converting between the mass of a solution and mass of solute when the mass percent concentration of a solution is known. The concentration of the KCN solution given in Part A corresponds to a mass percent of 0.173 %. What mass of a 0.173 % KCN solution contains 779 mg of KCN

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
You can practice converting between the mass of a solution and mass of solute when the mass percent...
Questions

Mathematics, 17.07.2019 18:40


Chemistry, 17.07.2019 18:40






English, 17.07.2019 18:40


Mathematics, 17.07.2019 18:40


Biology, 17.07.2019 18:40

Mathematics, 17.07.2019 18:40


English, 17.07.2019 18:40