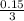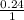Chemistry, 20.03.2020 02:58 vegeta8375
Two solutions are created by mixing one solution containing lithium nitrate with one containing sodium phosphate.
Solution A is created by mixing 0.500 L of 0.30 M LiNO3 with 0.400 L of 0.20 M Na3PO4.
Solution B is created by mixing 0.400 L of 0.20 M LiNO3 with 0.500 L of 0.30 M Na3PO4.
In which of these solutions will lithium phosphate precipitate? Lithium phosphate has a Ksp = 2.3x10−4 Select the solution(s) that will form a precipitate.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1


Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
You know the right answer?
Two solutions are created by mixing one solution containing lithium nitrate with one containing sodi...
Questions

Physics, 29.09.2019 18:10

Geography, 29.09.2019 18:10


Mathematics, 29.09.2019 18:10




Mathematics, 29.09.2019 18:10



English, 29.09.2019 18:10



History, 29.09.2019 18:10


Mathematics, 29.09.2019 18:10


Biology, 29.09.2019 18:10

Mathematics, 29.09.2019 18:10