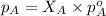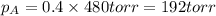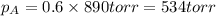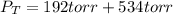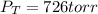Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
4. An ideal solution contains 40 mole percent of A and 60 mole percent of B at 80 oC. The vapor pres...
Questions

History, 12.07.2019 07:20

Social Studies, 12.07.2019 07:20


Biology, 12.07.2019 07:20

Biology, 12.07.2019 07:20






Physics, 12.07.2019 07:20

History, 12.07.2019 07:20

History, 12.07.2019 07:20





Health, 12.07.2019 07:20


Mathematics, 12.07.2019 07:20

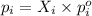
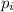 = vapor pressure of gas
= vapor pressure of gas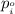 = vapor pressure of pure gas
= vapor pressure of pure gas 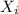 = mole fraction of gas
= mole fraction of gas