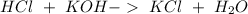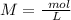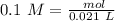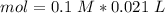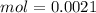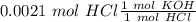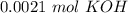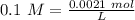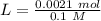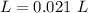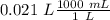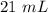Chemistry, 20.03.2020 03:34 emileehogan
Two 21.0 mL samples, one 0.100 M HCl and the other 0.100 M HF, were titrated with 0.200 M KOH. Answer each of the following questions regarding these two titrations. You may want to reference (Pages 755 - 769) Section 17.4 while completing this problem. Part A What is the volume of added base at the equivalence point for HCl

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2


You know the right answer?
Two 21.0 mL samples, one 0.100 M HCl and the other 0.100 M HF, were titrated with 0.200 M KOH. Answe...
Questions



Mathematics, 25.11.2021 14:00









Mathematics, 25.11.2021 14:00

Computers and Technology, 25.11.2021 14:00




Biology, 25.11.2021 14:00