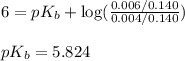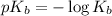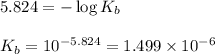Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

You know the right answer?
A student dissolves 0.0100 mole of an unknown weak base in 100.00 mL water and titrates the
s...
s...
Questions


English, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31

Health, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31

Biology, 14.12.2019 14:31



Biology, 14.12.2019 14:31

English, 14.12.2019 14:31

History, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31

Mathematics, 14.12.2019 14:31





History, 14.12.2019 14:31

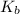 for weak base is
for weak base is 
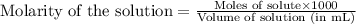
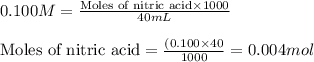
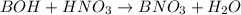
![pOH=pK_a+\log(\frac{[salt]}{[base]})](/tpl/images/0555/4585/13872.png)
![pOH=pK_b+\log(\frac{[BNO_3]}{[BOH]})](/tpl/images/0555/4585/b566f.png)
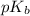 = negative logarithm of acid dissociation constant of formic acid = ?
= negative logarithm of acid dissociation constant of formic acid = ?
![[BNO_3]=\frac{0.004}{0.140}](/tpl/images/0555/4585/7cd20.png)
![[BOH]=\frac{0.006}{0.140} ](/tpl/images/0555/4585/f5b65.png)