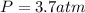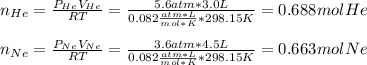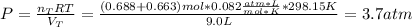Chemistry, 20.03.2020 04:20 GachaSkylarUwU
A sample of He gas (3.0 L) at 5.6 atm and 25°C was combined with 4.5 L of Ne gas at 3.6 atm and 25°C at constant temperature in a 9.0 L flask. The total pressure in the flask was atm. Assume the initial pressure in the flask was 0.00 atm and the temperature upon mixing was 25°C. Select one:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
A sample of He gas (3.0 L) at 5.6 atm and 25°C was combined with 4.5 L of Ne gas at 3.6 atm and 25°C...
Questions


Computers and Technology, 31.01.2021 05:50



English, 31.01.2021 05:50

Mathematics, 31.01.2021 05:50

Mathematics, 31.01.2021 05:50

Mathematics, 31.01.2021 05:50

Chemistry, 31.01.2021 05:50

Mathematics, 31.01.2021 05:50

Arts, 31.01.2021 05:50

Mathematics, 31.01.2021 05:50


Mathematics, 31.01.2021 05:50




Chemistry, 31.01.2021 06:00

Mathematics, 31.01.2021 06:00

Computers and Technology, 31.01.2021 06:00