Chemistry, 20.03.2020 05:50 jonystroyer1020
Given that a chlorine-oxygen bond has an enthalpy of 243 kJ/mol , an oxygen-oxygen bond has an enthalpy of 498 kJ/mol , and the standard enthalpy of formation of ClO2 102.5 kJ/mol , calculate the value for the enthalpy of formation per mole of ClO(g). What is the value for the enthalpy of formation per mole of ClO(g)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1


Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Given that a chlorine-oxygen bond has an enthalpy of 243 kJ/mol , an oxygen-oxygen bond has an entha...
Questions

Mathematics, 03.12.2020 01:20

History, 03.12.2020 01:20

Mathematics, 03.12.2020 01:20

Mathematics, 03.12.2020 01:20



Mathematics, 03.12.2020 01:20


Mathematics, 03.12.2020 01:20

Mathematics, 03.12.2020 01:20

History, 03.12.2020 01:20

History, 03.12.2020 01:20


Mathematics, 03.12.2020 01:20



Biology, 03.12.2020 01:20



Chemistry, 03.12.2020 01:20

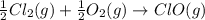
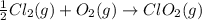 ;
; 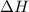 = 102.5 kJ
= 102.5 kJ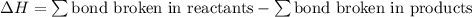
![[(\frac{1}{2})x + 498] - [(2)(243)]](/tpl/images/0555/5141/c814b.png)
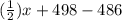
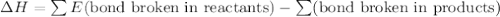
![[(\frac{1}{2})181 + (\frac{1}{2})498] - 243](/tpl/images/0555/5141/97422.png)