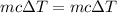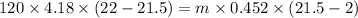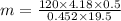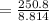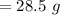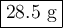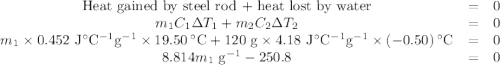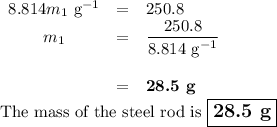Chemistry, 20.03.2020 05:58 soonerlady19
A volume of 120 mL of H2O is initially at room temperature (22.00 ∘C ). A chilled steel rod at 2.00 ∘C ∘C is placed in the water. If the final temperature of the system is 21.50 ∘C ∘C , what is the mass of the steel bar? Use the following values: specific heat of water = 4.18 J/(g⋅∘C)J/(g⋅∘C) specific heat of steel = 0.452 J/(g⋅∘C)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 23.06.2019 17:00
Identify the missing coefficient in the balanced equation and classify the type of reaction. mg(oh)2 + h2so4 ⟶ mgso4 + 1; combustion 1; neutralization 2; combustion 2; neutralization
Answers: 1

Chemistry, 23.06.2019 21:10
Which of the following characteristics are true about a typical peptide (amide) bond? the bond is planar. there is free rotation about the carbonyl carbon and nitrogen bond. there is substantial double-bond character to this bond. there is a net negative charge on nitrogen and net positive charge on oxygen.
Answers: 3
You know the right answer?
A volume of 120 mL of H2O is initially at room temperature (22.00 ∘C ). A chilled steel rod at 2.00...
Questions




Mathematics, 18.11.2020 19:00

History, 18.11.2020 19:00

Mathematics, 18.11.2020 19:00

Mathematics, 18.11.2020 19:00


Mathematics, 18.11.2020 19:00


Physics, 18.11.2020 19:00

History, 18.11.2020 19:00

Biology, 18.11.2020 19:00


English, 18.11.2020 19:00


Advanced Placement (AP), 18.11.2020 19:00

Spanish, 18.11.2020 19:00

Arts, 18.11.2020 19:00