Chemistry, 20.03.2020 06:29 lisafrench8222
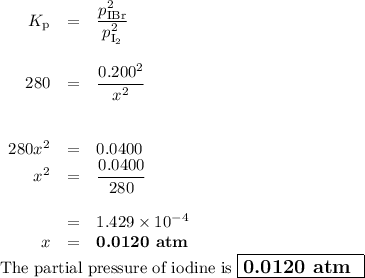
The equilibrium constant Kp for the reaction I2(g) + Br2(g) ⇀↽ 2 IBr(g) + 11.7 kJ is 280 at 150◦C. Suppose that a quantity of IBr is placed in a closed reaction vessel and the system is allowed to come to equilibrium at 150◦C. When equilibrium is established, the pressure of IBr is 0.200 atm. What is the pressure of I2 at equilibrium?

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2
You know the right answer?
The equilibrium constant Kp for the reaction I2(g) + Br2(g) ⇀↽ 2 IBr(g) + 11.7 kJ is 280 at 150◦C. S...
Questions

Arts, 06.05.2020 20:14



Social Studies, 06.05.2020 20:14










History, 06.05.2020 20:14






History, 06.05.2020 20:14

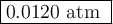
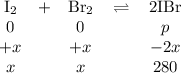
 }
}