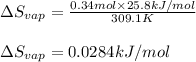Chemistry, 20.03.2020 07:24 ramanpreet
The molar heat of vaporization of pentane is 25.8 kJ·mol−1, and the boiling point of pentane is 36.1°C. Calculate the value of ΔvapS for the vaporization of 0.34 mole of pentane.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
The molar heat of vaporization of pentane is 25.8 kJ·mol−1, and the boiling point of pentane is 36.1...
Questions


Mathematics, 27.01.2021 18:30


Mathematics, 27.01.2021 18:30


Mathematics, 27.01.2021 18:30

Chemistry, 27.01.2021 18:30


Computers and Technology, 27.01.2021 18:30


Mathematics, 27.01.2021 18:30

Chemistry, 27.01.2021 18:30

Mathematics, 27.01.2021 18:30




Mathematics, 27.01.2021 18:30

Mathematics, 27.01.2021 18:30


Computers and Technology, 27.01.2021 18:30

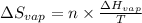
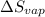 = Entropy change of vaporization = ?
= Entropy change of vaporization = ?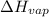 = molar heat of vaporization = 25.8 kJ/mol
= molar heat of vaporization = 25.8 kJ/mol![36.1^oC=[36.1+273]K=309.1K](/tpl/images/0555/6193/0c07c.png)