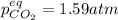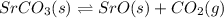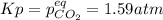Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2


Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

You know the right answer?
For the reaction below, Kp = 1.59 at 100°C. If 1.0 g of SrCO3 is placed in an empty 5.00 L reactor a...
Questions

English, 19.05.2020 03:24

Biology, 19.05.2020 03:24

English, 19.05.2020 03:24

History, 19.05.2020 03:24


History, 19.05.2020 03:24

Mathematics, 19.05.2020 03:24

Spanish, 19.05.2020 03:24



Mathematics, 19.05.2020 03:24

Mathematics, 19.05.2020 03:24


History, 19.05.2020 03:24


History, 19.05.2020 03:24

Mathematics, 19.05.2020 03:24

Health, 19.05.2020 03:24

Mathematics, 19.05.2020 03:24