
Chemistry, 20.03.2020 08:30 darwin59651
Calculate the pH of a 0.437 M aqueous solution of hydrocyanic acid (HCN, Ka = 4.0×10-10) and the equilibrium concentrations of the weak acid and its conjugate base.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
The wilson chamber is used to study: direction, speed, and distance of radioactivity the intensity of radiation the appearance of individual atoms all of the above
Answers: 1

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1
You know the right answer?
Calculate the pH of a 0.437 M aqueous solution of hydrocyanic acid (HCN, Ka = 4.0×10-10) and the equ...
Questions

Advanced Placement (AP), 28.12.2020 14:00

Mathematics, 28.12.2020 14:00

Business, 28.12.2020 14:00

Arts, 28.12.2020 14:00

English, 28.12.2020 14:00



English, 28.12.2020 14:00





Geography, 28.12.2020 14:00


Computers and Technology, 28.12.2020 14:00



English, 28.12.2020 14:00


Chemistry, 28.12.2020 14:00

![[HCN]_{eq}=0.43699M](/tpl/images/0555/6850/8a21f.png)
![[CN^-]_{eq}=1.322x10^{-5}M](/tpl/images/0555/6850/83c77.png)
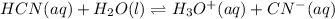
![Ka=\frac{[H^+]_{eq}[CN^-]_{eq}}{[HCN]_{eq}}](/tpl/images/0555/6850/4425b.png)
 due to the reaction extent, goes:
due to the reaction extent, goes: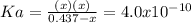
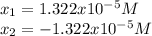
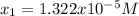
![pH=-log([H^+])=-log(1.322x10^{-5})=4.88](/tpl/images/0555/6850/f810b.png)
![[HCN]_{eq}=0.437M-1.322x10^{-5}M=0.43699M](/tpl/images/0555/6850/d4f3f.png)


