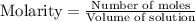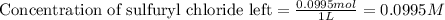Chemistry, 20.03.2020 09:55 solobiancaa
The decomposition of sulfuryl chloride into sulfur dioxide and chlorine SO2Cl2(g) → SO2(g) + Cl2(g) follows first-order kinetics. At 320◦C the rate constant is 2.2 × 10−5 sec−1 . If one started with a sample containing 0.16 moles of sulfuryl chloride per liter at 320◦C, what concentration would be left after 6.00 hours?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3
You know the right answer?
The decomposition of sulfuryl chloride into sulfur dioxide and chlorine SO2Cl2(g) → SO2(g) + Cl2(g)...
Questions


Mathematics, 26.10.2021 09:30


Mathematics, 26.10.2021 09:30


Biology, 26.10.2021 09:30

Medicine, 26.10.2021 09:30



History, 26.10.2021 09:30

Computers and Technology, 26.10.2021 09:30

History, 26.10.2021 09:30


English, 26.10.2021 09:30






Computers and Technology, 26.10.2021 09:30

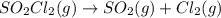
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0555/8016/f1041.png)
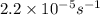
![[A_o]](/tpl/images/0555/8016/dc622.png) = initial amount of the sample = 0.16 moles
= initial amount of the sample = 0.16 moles![2.2\times 10^{-5}=\frac{2.303}{21600}\log\frac{0.16}{[A]}](/tpl/images/0555/8016/ba4a8.png)
![[A]=0.0995moles](/tpl/images/0555/8016/152d1.png)