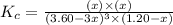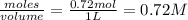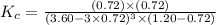Chemistry, 20.03.2020 10:09 poptropic7932
Carbon tetrachloride can be produced by the following reaction: Suppose 1.20 mol of and 3.60 mol of were placed in a 1.00-L flask at an unknown temperature. After equilibrium has been achieved, the mixture contains 0.72 mol . Calculate at the unknown temperature.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3
You know the right answer?
Carbon tetrachloride can be produced by the following reaction: Suppose 1.20 mol of and 3.60 mol of...
Questions

Business, 16.08.2021 18:40



Chemistry, 16.08.2021 18:40


Mathematics, 16.08.2021 18:40


Mathematics, 16.08.2021 18:40

English, 16.08.2021 18:40

Mathematics, 16.08.2021 18:40







Chemistry, 16.08.2021 18:40



Mathematics, 16.08.2021 18:40

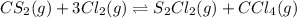
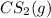 of and 3.60 mol of
of and 3.60 mol of 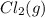 were placed in a 1.00-L flask at an unknown temperature. After equilibrium has been achieved, the mixture contains 0.72 mol of
were placed in a 1.00-L flask at an unknown temperature. After equilibrium has been achieved, the mixture contains 0.72 mol of 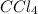 . Calculate equilibrium constant at the unknown temperature.
. Calculate equilibrium constant at the unknown temperature.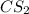 = 1.20 mole
= 1.20 mole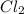 = 3.60 mole
= 3.60 mole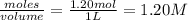
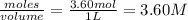
![K_c=\frac{[S_2Cl_2]\times [CCl_4]}{[Cl_2]^3[CS_2]}](/tpl/images/0555/8680/e9703.png)