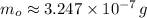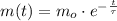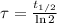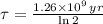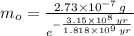Chemistry, 20.03.2020 09:44 leonardkaren41ovlx1q
Geologists use the decay of potassium-40 in volcanic rocks to determine their age. Potassium-40 has a half-life of 1.26 109 years, so it can be used to date very old rocks. If a sample of rock 3.15 108 years old contains 2.73 10-7 g of potassium-40 today, how much potassium-40 was originally present in the rock

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2


Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
Geologists use the decay of potassium-40 in volcanic rocks to determine their age. Potassium-40 has...
Questions

History, 02.08.2019 19:00

History, 02.08.2019 19:00


English, 02.08.2019 19:00




History, 02.08.2019 19:00

History, 02.08.2019 19:00

Business, 02.08.2019 19:00

Biology, 02.08.2019 19:00

Mathematics, 02.08.2019 19:00

Physics, 02.08.2019 19:00


Spanish, 02.08.2019 19:00

History, 02.08.2019 19:00

Mathematics, 02.08.2019 19:00


Computers and Technology, 02.08.2019 19:00