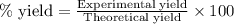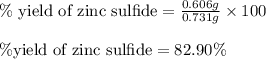Chemistry, 20.03.2020 09:51 grangian06
Determine the theoretical yield of ZnS that can be prepared from the reaction of 0.488 g Zn with 0.503 g S8. What is the percent yield, if 0.606 g of ZnS were experimentally recovered

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 23.06.2019 10:30
Ireally need ! calcium metal reacts with a potassium chloride solution to form calcium chloride and potassium ions. balance this reaction. (s) + (aq) → cacl2(s) + +(aq) a) 1, 2, 1, 2 b) 1, 2, 1, 1 c) 1, 1, 1, 1 d) 2, 1, 2, 1
Answers: 1
You know the right answer?
Determine the theoretical yield of ZnS that can be prepared from the reaction of 0.488 g Zn with 0.5...
Questions



Mathematics, 02.06.2021 04:10

Mathematics, 02.06.2021 04:10

Mathematics, 02.06.2021 04:10

Mathematics, 02.06.2021 04:10




Mathematics, 02.06.2021 04:10


Computers and Technology, 02.06.2021 04:10



Mathematics, 02.06.2021 04:10


History, 02.06.2021 04:10



Mathematics, 02.06.2021 04:10

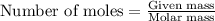 .....(1)
.....(1)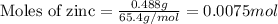
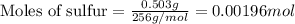
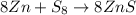
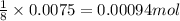 of sulfur
of sulfur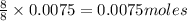 of zinc sulfide
of zinc sulfide