Chemistry, 20.03.2020 09:52 anggelkevin100
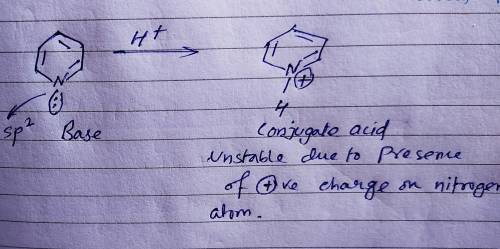
Which of the following bases is the WEAKEST? The base is followed by its Kb value. Which of the following bases is the WEAKEST? The base is followed by its Kb value. (CH3CH2)3N, 5.2 × 10-4 C5H5N, 1.7 × 10-9 NH3, 1.76 × 10-5 HOCH2CH2NH2, 3.2 × 10-5 Since these are all weak bases, they have the same strength.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3


Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
Which of the following bases is the WEAKEST? The base is followed by its Kb value. Which of the foll...
Questions

Mathematics, 19.04.2021 03:50

Mathematics, 19.04.2021 03:50



Mathematics, 19.04.2021 03:50




Mathematics, 19.04.2021 03:50

Mathematics, 19.04.2021 03:50

Mathematics, 19.04.2021 03:50

Spanish, 19.04.2021 03:50

English, 19.04.2021 03:50

Business, 19.04.2021 03:50

Chemistry, 19.04.2021 03:50

English, 19.04.2021 03:50

Mathematics, 19.04.2021 03:50


Mathematics, 19.04.2021 03:50

Mathematics, 19.04.2021 03:50