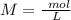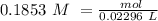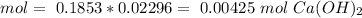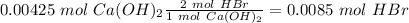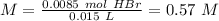Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1


Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1
You know the right answer?
The titration of 15.00 mL of hydrobromic acid required 22.96 mL of a 0.1853 M calcium hydroxide to r...
Questions



Business, 12.09.2021 14:00


Health, 12.09.2021 14:00


Chemistry, 12.09.2021 14:00

English, 12.09.2021 14:00


English, 12.09.2021 14:00

Mathematics, 12.09.2021 14:00


Mathematics, 12.09.2021 14:00

Mathematics, 12.09.2021 14:00

History, 12.09.2021 14:00



History, 12.09.2021 14:00

Chemistry, 12.09.2021 14:00

Engineering, 12.09.2021 14:00