
Chemistry, 20.03.2020 10:06 masteroftheuniverse3
Solving for the pH in a mixture of acids is like dealing with a diprotic acid. You solve the problem by dealing with the acids in successive order. You start with the stronger acid (just like you start with the Ka1 because it is higher than the Ka2 for a diprotic). Afterward, you move on to the weaker acid. If a solution contains 0.029 M HCl and 0.100 M HF (Ka=6.6x10-4), what will be the pH of the solution?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1


Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
Solving for the pH in a mixture of acids is like dealing with a diprotic acid. You solve the problem...
Questions

History, 25.08.2019 03:30

Social Studies, 25.08.2019 03:30


Biology, 25.08.2019 03:30

Mathematics, 25.08.2019 03:30

History, 25.08.2019 03:30

Mathematics, 25.08.2019 03:30



Mathematics, 25.08.2019 03:30

Biology, 25.08.2019 03:30

Social Studies, 25.08.2019 03:30

Mathematics, 25.08.2019 03:30

Mathematics, 25.08.2019 03:30

English, 25.08.2019 03:30




History, 25.08.2019 03:30

Mathematics, 25.08.2019 03:30

![[H^+]](/tpl/images/0555/8561/07acb.png)
![[HCl]=[H^+]=0.029 M](/tpl/images/0555/8561/09dd5.png)
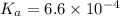
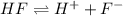
![K_a=\frac{[H^+][F^-]}{[HF]}=\frac{x\times x}{(c-x)}](/tpl/images/0555/8561/1ea44.png)
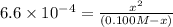
![[H^+]'=x=0.0078 M](/tpl/images/0555/8561/68e8c.png)
![[H^+]_t=[H^+]+[H^+]'=0.029 M + 0.0078 M=0.0368 M](/tpl/images/0555/8561/81922.png)
![pH=-\log[H^+]_t](/tpl/images/0555/8561/ac0ab.png)
![=-\log[0.0368 M]=1.43](/tpl/images/0555/8561/d2fa1.png)



