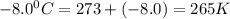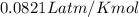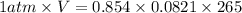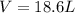Chemistry, 20.03.2020 10:04 brandon56238
A reaction at −8.0°C evolves 854.mmol of boron trifluoride gas. Calculate the volume of boron trifluoride gas that is collected. You can assume the pressure in the room is exactly 1atm. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
A reaction at −8.0°C evolves 854.mmol of boron trifluoride gas. Calculate the volume of boron triflu...
Questions

History, 16.07.2019 11:30


English, 16.07.2019 11:30

Mathematics, 16.07.2019 11:30

Biology, 16.07.2019 11:30



History, 16.07.2019 11:30

History, 16.07.2019 11:30

Computers and Technology, 16.07.2019 11:30



Social Studies, 16.07.2019 11:30


History, 16.07.2019 11:30

Computers and Technology, 16.07.2019 11:30

Mathematics, 16.07.2019 11:30

Mathematics, 16.07.2019 11:30