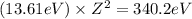Chemistry, 20.03.2020 10:04 morkitus13
An ion Mn+ has a single electron. The highest energy line in its emission spectrum occurs at a frequency of 8.225 × 1016 Hz. Identify the ion. (Enter the symbol of the element in the first box, and its charge in the second.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1


Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

You know the right answer?
An ion Mn+ has a single electron. The highest energy line in its emission spectrum occurs at a frequ...
Questions


English, 28.09.2020 14:01

Advanced Placement (AP), 28.09.2020 14:01



Chemistry, 28.09.2020 14:01



Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01






Mathematics, 28.09.2020 14:01




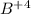
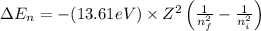
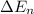 = change in energy
= change in energy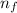 = Higher energy level =
= Higher energy level = 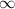
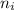 = Lower energy level = 1
= Lower energy level = 1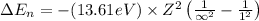
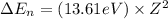 ............(1)
............(1)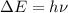
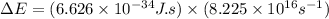
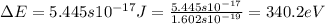 .......(2)
.......(2)