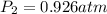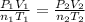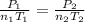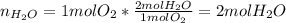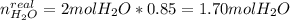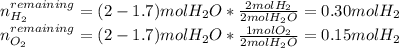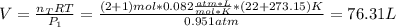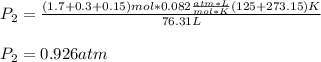A mixture in which the mole ratio of hydrogen to oxygen is (exactly) 2:1 is used to prepare water by the reaction 2 H2 (g) + O2 (g) → 2 H2O (g) The total pressure in the container is 0.951 atm at 22°C before the reaction. What is the final pressure in the container after the reaction, with a final temperature of 125°C, no volume change, and an 85.0% yield?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
A mixture in which the mole ratio of hydrogen to oxygen is (exactly) 2:1 is used to prepare water by...
Questions

Mathematics, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00


Health, 18.12.2020 14:00

Chemistry, 18.12.2020 14:00


Physics, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00

History, 18.12.2020 14:00

Advanced Placement (AP), 18.12.2020 14:00


Mathematics, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00

History, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00


History, 18.12.2020 14:00

English, 18.12.2020 14:00