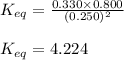Chemistry, 20.03.2020 10:11 sosick90501
For the reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) the equilibrium concentrations were found to be [ H 2 O ] = 0.250 M , [ H 2 ] = 0.330 M , and [ O 2 ] = 0.800 M . What is the equilibrium constant for this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1


Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
For the reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) the equilibrium concentrations were f...
Questions

Mathematics, 29.09.2019 02:30

Geography, 29.09.2019 02:30

History, 29.09.2019 02:30

Mathematics, 29.09.2019 02:30

Mathematics, 29.09.2019 02:30

English, 29.09.2019 02:30

Business, 29.09.2019 02:30

Mathematics, 29.09.2019 02:30


Social Studies, 29.09.2019 02:30



Biology, 29.09.2019 02:30


English, 29.09.2019 02:30

History, 29.09.2019 02:30

English, 29.09.2019 02:30


Mathematics, 29.09.2019 02:30

Social Studies, 29.09.2019 02:30

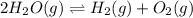
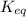 for above reaction follows:
for above reaction follows:![K_{eq}=\frac{[H_2][O_2]}{[H_2O]^2}](/tpl/images/0555/8774/3db32.png)