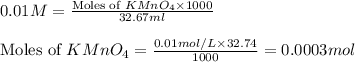Chemistry, 20.03.2020 10:46 jdisalle476
A sample of an Iron Oxalato complex salt weighting 0.13 grams requires 32.74 mL of 0.01 M KMnO4 to turn the solution a very light pink color at the quivalence point. Calculate the number of moles of KMnO4 added.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
A sample of an Iron Oxalato complex salt weighting 0.13 grams requires 32.74 mL of 0.01 M KMnO4 to t...
Questions

History, 08.04.2020 00:19

History, 08.04.2020 00:19


Computers and Technology, 08.04.2020 00:19


Mathematics, 08.04.2020 00:19







Computers and Technology, 08.04.2020 00:19


Computers and Technology, 08.04.2020 00:19

Social Studies, 08.04.2020 00:19




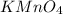 added are 0.0003
added are 0.0003 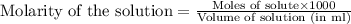 .....(1)
.....(1)