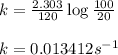Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
For a first-order reaction, after 2.00 min, 20% of the reactants remain. Calculate the rate constant...
Questions

History, 21.05.2020 19:10


Mathematics, 21.05.2020 19:10

English, 21.05.2020 19:10

Mathematics, 21.05.2020 19:10

Mathematics, 21.05.2020 19:10




Mathematics, 21.05.2020 19:10


English, 21.05.2020 19:10




Mathematics, 21.05.2020 19:10


History, 21.05.2020 19:10

History, 21.05.2020 19:10

Mathematics, 21.05.2020 19:10


![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0556/0145/f1041.png)
![[A_o]](/tpl/images/0556/0145/dc622.png) = initial amount of the sample = 100 grams
= initial amount of the sample = 100 grams