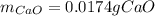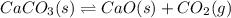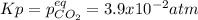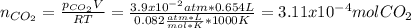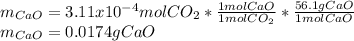Chemistry, 20.03.2020 11:04 Affousietta
A sample of CaCO3(s) is introduced into a sealed container of volume 0.654 L and heated to 1000 K until equilibrium is reached. The Kp for the reaction CaCO3(s) ∆ CaO(s) + CO2(g) is 3.9 * 10-2 at this temperature. Calculate the mass of CaO(s) that is present at equilibriu

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
A sample of CaCO3(s) is introduced into a sealed container of volume 0.654 L and heated to 1000 K un...
Questions


Mathematics, 12.02.2021 09:20

English, 12.02.2021 09:20

Social Studies, 12.02.2021 09:20



Mathematics, 12.02.2021 09:20

Mathematics, 12.02.2021 09:20

Mathematics, 12.02.2021 09:20



Chemistry, 12.02.2021 09:20




Social Studies, 12.02.2021 09:20

Mathematics, 12.02.2021 09:20


Mathematics, 12.02.2021 09:20