
Chemistry, 20.03.2020 11:14 ciarrap552
If you assume this reaction is driven to completion because of the large excess of one ion, what is the concentration of [Fe(SCN)]2+ that would be formed from 6.00 mL of 0.00180 M KSCN 5.00 mL 0.240 M Fe(NO3)3 and 14.00 mL of 0.050 M HNO3.
Question 3 options:
0.240 M
4.32 x 10^-4
0.0480
0.0460 M

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
If you assume this reaction is driven to completion because of the large excess of one ion, what is...
Questions

Mathematics, 13.12.2021 22:20

Mathematics, 13.12.2021 22:20


Mathematics, 13.12.2021 22:20


Business, 13.12.2021 22:20






Mathematics, 13.12.2021 22:20

Physics, 13.12.2021 22:20

Mathematics, 13.12.2021 22:20

Mathematics, 13.12.2021 22:20

World Languages, 13.12.2021 22:20


Mathematics, 13.12.2021 22:20


![[Fe(SCN)]^{2+}](/tpl/images/0556/0307/0c409.png) is,
is, 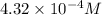
 and
and 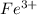 is excess reagent.
is excess reagent.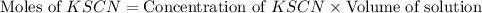

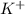 = Moles of
= Moles of 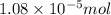
![\text{Concentration of }[Fe(SCN)]^{2+}=\frac{\text{Moles of }[Fe(SCN)]^{2+}}{\text{Volume of solution}}](/tpl/images/0556/0307/e16b4.png)
![\text{Concentration of }[Fe(SCN)]^{2+}=\frac{1.08\times 10^{-5}mol}{0.025L}=4.32\times 10^{-4}M](/tpl/images/0556/0307/cd669.png)


