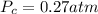Chemistry, 20.03.2020 11:59 martinezzz2294
1.00 mole of an ideal monoatomic gas at STP first undergoes isothermal expansion so that the volume at b is 2.5 times the volume at a. Next, heat is extracted at a constant volume so that the pressure drops. The gas is then compressed adiabatically back to the original state. Calculate the pressure at c.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 23.06.2019 10:00
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1

Chemistry, 23.06.2019 23:30
Which of the following is not a difference between a compound and a mixture? 1 some mixtures are homogenous, while others are heterogeneous. all compounds are homogeneous. 2 mixtures are homogeneous while compounds are heterogeneous. 3 no chemical bonding occurs between the components of a mixture. the properties of atoms and molecules are not changed when they become part of a mixture. 3 mixtures can be separated by physical means, for example, straining, filtering, or evaporation. 4compounds can only be separated into their constituent atoms by chemically breaking bonds.
Answers: 2
You know the right answer?
1.00 mole of an ideal monoatomic gas at STP first undergoes isothermal expansion so that the volume...
Questions

Mathematics, 28.06.2019 06:30

History, 28.06.2019 06:30

Mathematics, 28.06.2019 06:30




History, 28.06.2019 06:30

Chemistry, 28.06.2019 06:30

Mathematics, 28.06.2019 06:30


English, 28.06.2019 06:30



History, 28.06.2019 06:30






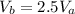
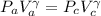
![\frac{P_c}{P_a}=[\frac{V_a}{V_c}]^{(2/3)](/tpl/images/0556/1040/0de96.png)
![\frac{P_c}{1.0 atm}=[\frac{1}{2.5}]^{(2/3)](/tpl/images/0556/1040/c3aec.png)