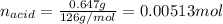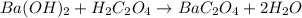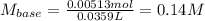Chemistry, 20.03.2020 11:54 jadenpmoore2008
Oxalic acid dihydrate is a solid, diprotic acid that can be used in the laboratory as a primary standard. Its formula is H2C2O4•2H2O. A student dissolves 0.647 grams of H2C2O4•2H2O in water and titrates the resulting solution with a solution of barium hydroxide of unknown concentration. If 35.9 mL of the barium hydroxide solution are required to neutralize the acid, what is the molarity of the barium hydroxide solution ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 06:30
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3
You know the right answer?
Oxalic acid dihydrate is a solid, diprotic acid that can be used in the laboratory as a primary stan...
Questions


Biology, 18.01.2021 04:20


Mathematics, 18.01.2021 04:20

History, 18.01.2021 04:20

English, 18.01.2021 04:20

English, 18.01.2021 04:20


Mathematics, 18.01.2021 04:20


Mathematics, 18.01.2021 04:20


Mathematics, 18.01.2021 04:20

Mathematics, 18.01.2021 04:20

Mathematics, 18.01.2021 04:20




Mathematics, 18.01.2021 04:20

Mathematics, 18.01.2021 04:20