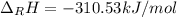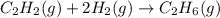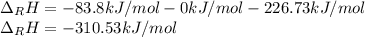Chemistry, 20.03.2020 12:02 tae8002001
Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ/mole) the standard enthalpy change ΔH° for the hydrogenation of ethyne (acetylene) to ethane. Just enter a number (no units).

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ/mole)...
Questions

Mathematics, 19.01.2021 08:00

Social Studies, 19.01.2021 08:00



Mathematics, 19.01.2021 08:00



Mathematics, 19.01.2021 08:00







Social Studies, 19.01.2021 08:00

Mathematics, 19.01.2021 08:00

Mathematics, 19.01.2021 08:00


Mathematics, 19.01.2021 08:00