
Chemistry, 20.03.2020 12:02 PAADUUUgma
Consider the first-order reaction described by the equation At a certain temperature, the rate constant for this reaction is 5.82 × 10 − 4 s − 1 5.82×10−4 s−1 . Calculate the half-life of cyclopropane at this temperature.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2
You know the right answer?
Consider the first-order reaction described by the equation At a certain temperature, the rate const...
Questions

Mathematics, 12.03.2021 01:00




Social Studies, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00


English, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00

History, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00




Physics, 12.03.2021 01:00

History, 12.03.2021 01:00




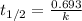
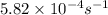
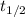 = half life of the reaction = ?
= half life of the reaction = ?


