
Chemistry, 20.03.2020 12:03 cedricevans41p4j3kx
A 140.0-g sample of water at 25.0°C is mixed with 108.6 g of a certain metal at 100.0°C. After thermal equilibrium is established, the (final) temperature of the mixture is 29.6°C. What is the specific heat capacity of the metal, assuming it is constant over the temperature range concerned?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1


You know the right answer?
A 140.0-g sample of water at 25.0°C is mixed with 108.6 g of a certain metal at 100.0°C. After therm...
Questions

Computers and Technology, 24.07.2021 09:00



Health, 24.07.2021 09:00

Mathematics, 24.07.2021 09:00

Mathematics, 24.07.2021 09:00


Computers and Technology, 24.07.2021 09:00

Mathematics, 24.07.2021 09:00

English, 24.07.2021 09:00

Social Studies, 24.07.2021 09:00


Health, 24.07.2021 09:20


Chemistry, 24.07.2021 09:20


Mathematics, 24.07.2021 09:20



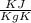
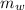 = 140 gm = 0.14 kg
= 140 gm = 0.14 kg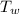 = 25°c = 298 K
= 25°c = 298 K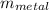 = 108.6 gm = 0.1086 kg
= 108.6 gm = 0.1086 kg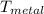 = 100°c = 373 K
= 100°c = 373 K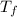 = 29.6°c = 302.6 K
= 29.6°c = 302.6 K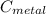 × (
× ( 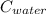 × (
× ( 

