
Pure copper may be produced by the reaction of copper(I) sulfide with oxygen gas as follows: Cu2S(s) + O2(g) 2Cu(s) + SO2(g) If 0.680 kg of copper(I) sulfide reacts with excess oxygen, what mass of copper metal may be produced ? A) 0.680 kg B) 0.136 kg C) 0.271 kg D) 0.543 kg E) 1.36 kg

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2



Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
Pure copper may be produced by the reaction of copper(I) sulfide with oxygen gas as follows: Cu2S(s)...
Questions


English, 22.09.2019 22:00


Social Studies, 22.09.2019 22:00





Social Studies, 22.09.2019 22:00

Biology, 22.09.2019 22:00

Physics, 22.09.2019 22:00


Mathematics, 22.09.2019 22:00

Biology, 22.09.2019 22:00

Health, 22.09.2019 22:00


Physics, 22.09.2019 22:00



Computers and Technology, 22.09.2019 22:00

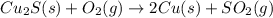
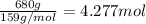
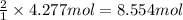 copper
copper

