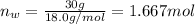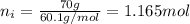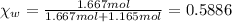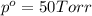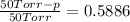Chemistry, 20.03.2020 12:59 Hannahmiller3773
What is the vapor pressure of isopropanol (MW = 60.1) in a closed container of 70% isopropanol in water (MW = 18.0) at 25oC? Such a mixture contains 70 g of isopropanol and 30 g of water. The vapor pressure of pure isopropanol at 25oC = 50 torr

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

You know the right answer?
What is the vapor pressure of isopropanol (MW = 60.1) in a closed container of 70% isopropanol in wa...
Questions


Mathematics, 19.02.2020 10:54

Geography, 19.02.2020 10:54

Health, 19.02.2020 10:54

Mathematics, 19.02.2020 10:55

Health, 19.02.2020 10:56

Chemistry, 19.02.2020 10:57


Mathematics, 19.02.2020 10:59

Mathematics, 19.02.2020 11:01

Chemistry, 19.02.2020 11:02


Biology, 19.02.2020 11:06




Mathematics, 19.02.2020 11:10

Mathematics, 19.02.2020 11:10

History, 19.02.2020 11:11

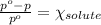
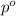 = Vapor pressure of pure solvent
= Vapor pressure of pure solvent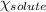 = Mole fraction of solute
= Mole fraction of solute