
Chemistry, 31.08.2019 03:50 Lucialari4345
How is electronegativity related to covalent bonding?
atoms must have similar electronegativities in order to share electrons in a covalent bond.
atoms must have very different electronegativities in order to transfer electrons in a covalent bond.
atoms must have similar electronegativities in order to transfer electrons in a covalent bond.
atoms must have very different electronegativities in order to share electrons in a covalent bond.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
How is electronegativity related to covalent bonding?
atoms must have similar electronegativi...
atoms must have similar electronegativi...
Questions

History, 21.01.2021 18:30

Chemistry, 21.01.2021 18:30

Mathematics, 21.01.2021 18:30

Arts, 21.01.2021 18:30


English, 21.01.2021 18:30

Computers and Technology, 21.01.2021 18:30

Business, 21.01.2021 18:30


Mathematics, 21.01.2021 18:30


Social Studies, 21.01.2021 18:30


Biology, 21.01.2021 18:30

Social Studies, 21.01.2021 18:30


Mathematics, 21.01.2021 18:30

Mathematics, 21.01.2021 18:30



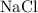 molecule the electronegativity difference is approximately 2.1. Whenever the difference exceeds 1.8 the bonding is regarded as ionic. This is the reason that sodium chloride is an ionic compound.
molecule the electronegativity difference is approximately 2.1. Whenever the difference exceeds 1.8 the bonding is regarded as ionic. This is the reason that sodium chloride is an ionic compound.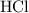 . Here the bond is polar but the difference in electronegativity does not exceed 1.8 and hence it is regarded as covalent molecule.
. Here the bond is polar but the difference in electronegativity does not exceed 1.8 and hence it is regarded as covalent molecule.

