
Chemistry, 21.03.2020 02:02 daartist3121
2 H2+O2=2 H2O
if 100 grams of oxygen gas are used what would the percent yield be if 75g of H2O was produced?
Show your work.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1



Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
2 H2+O2=2 H2O
if 100 grams of oxygen gas are used what would the percent yield be if 75g of H...
if 100 grams of oxygen gas are used what would the percent yield be if 75g of H...
Questions

Health, 06.05.2020 08:13

Biology, 06.05.2020 08:13

Chemistry, 06.05.2020 08:13


Chemistry, 06.05.2020 08:13

Mathematics, 06.05.2020 08:13

Mathematics, 06.05.2020 08:13



Spanish, 06.05.2020 08:13


History, 06.05.2020 08:13

Mathematics, 06.05.2020 08:13



Biology, 06.05.2020 08:13

Mathematics, 06.05.2020 08:13



Mathematics, 06.05.2020 08:13

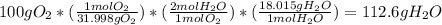
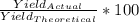 =
= 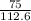 = 66.60403646
= 66.60403646


