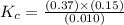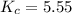Chemistry, 21.03.2020 02:58 alexisfaithsmith
At the equilibrium point in the decomposition of phosphorus pentachloride to chlorine and phosphorus trichloride, the following concentrations are obtained: 0.010 mol/L PCl5, 0.15 mol/l PCl3 and 0.37 mol/L Cl2. Determine the Keq for the reaction

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What type of reaction is represented by the following example? 2co2 (g) + 4h2o (l) + 1452 kj 2ch3oh (l) (g) + 3o2 (g) exothermic endothermic
Answers: 1

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1
You know the right answer?
At the equilibrium point in the decomposition of phosphorus pentachloride to chlorine and phosphorus...
Questions


History, 23.01.2020 02:31

Mathematics, 23.01.2020 02:31



Mathematics, 23.01.2020 02:31

History, 23.01.2020 02:31

History, 23.01.2020 02:31



Mathematics, 23.01.2020 02:31

Mathematics, 23.01.2020 02:31


English, 23.01.2020 02:31


History, 23.01.2020 02:31


Health, 23.01.2020 02:31

Mathematics, 23.01.2020 02:31

Mathematics, 23.01.2020 02:31

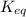 for the reaction is 5.55
for the reaction is 5.55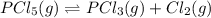
![K_c=\frac{[Cl_2]\times [PCl_3]}{[PCl_5]}](/tpl/images/0557/1142/ffe89.png)