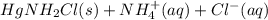Chemistry, 21.03.2020 03:24 edmundg2238
To your unknown you add HCl to precipitate the Group A ions (Ag and Hg). This precipitate is removed and NH3 is added to it to redissolve the Ag and check for the presence of Hg. From the picture below which was taken right after the NH3 was added, please select a choice that best represents what you are seeing.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2

Chemistry, 23.06.2019 02:00
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
You know the right answer?
To your unknown you add HCl to precipitate the Group A ions (Ag and Hg). This precipitate is removed...
Questions

English, 29.01.2020 13:53

History, 29.01.2020 13:53

Mathematics, 29.01.2020 13:53



English, 29.01.2020 13:53

Mathematics, 29.01.2020 13:53


History, 29.01.2020 13:53

Social Studies, 29.01.2020 13:53

English, 29.01.2020 13:53


Spanish, 29.01.2020 13:53

Social Studies, 29.01.2020 13:53

Mathematics, 29.01.2020 13:53



Physics, 29.01.2020 13:53

Biology, 29.01.2020 13:53

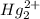 (aq) + 2Cl- →
(aq) + 2Cl- → 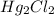 (s)
(s)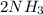 (aq) →
(aq) → 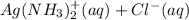
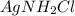 :
: