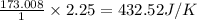Chemistry, 21.03.2020 04:26 zitterkoph
Consider the reaction 2CO(g) + O2(g)2CO2(g) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 2.25 moles of CO(g) react at standard conditions. S°surroundings = J/K

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
Consider the reaction 2CO(g) + O2(g)2CO2(g) Using standard thermodynamic data at 298K, calculate the...
Questions

History, 06.05.2021 01:00


Mathematics, 06.05.2021 01:00

History, 06.05.2021 01:00




Chemistry, 06.05.2021 01:00

Mathematics, 06.05.2021 01:00


Mathematics, 06.05.2021 01:00

Mathematics, 06.05.2021 01:00


Chemistry, 06.05.2021 01:00



Advanced Placement (AP), 06.05.2021 01:00

Mathematics, 06.05.2021 01:00


Computers and Technology, 06.05.2021 01:00

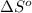 for the surrounding when given amount of CO is reacted is 432.52 J/K
for the surrounding when given amount of CO is reacted is 432.52 J/K![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{(product)}]-\sum [n\times \Delta S^o_{(reactant)}]](/tpl/images/0557/2961/52737.png)
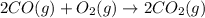
![\Delta S^o_{rxn}=[(2\times \Delta S^o_{(CO_2(g))})]-[(1\times \Delta S^o_{(O_2(g))})+(2\times \Delta S^o_{(CO(g))})]](/tpl/images/0557/2961/30479.png)
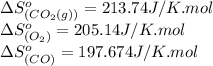
![\Delta S^o_{rxn}=[(2\times (213.74))]-[(1\times (205.14))+(2\times (197.674))]\\\\\Delta S^o_{rxn}=-173.008J/K](/tpl/images/0557/2961/a0064.png)