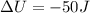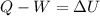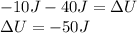Chemistry, 21.03.2020 06:04 jameslinimk
A sample of gas is placed in an ice bath so that there is 10 J of heat transfer out of the sample. At the same time, the gas is compressed by a piston that does 40 J of Work on the gas. What is the change in thermal energy of the gas

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

You know the right answer?
A sample of gas is placed in an ice bath so that there is 10 J of heat transfer out of the sample. A...
Questions

Mathematics, 28.06.2021 01:20


History, 28.06.2021 01:20


Mathematics, 28.06.2021 01:30

Computers and Technology, 28.06.2021 01:30



Mathematics, 28.06.2021 01:30






Mathematics, 28.06.2021 01:30


Mathematics, 28.06.2021 01:30

English, 28.06.2021 01:30

Mathematics, 28.06.2021 01:30