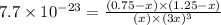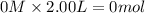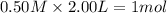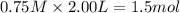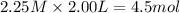Chemistry, 21.03.2020 07:59 louieknown
Consider the reaction CO(g) + 3 H2 (g) ⇌ CH4 (g) + H2O(g) Kc = 7.7×10–23 at 25 °C. 1.50 mol CH4 and 2.50 mol H2O are added to an empty 2.00-L container at 25 °C and allowed to reach equilibrium. What are the equilibrium amounts (in moles) of each species? 1.5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
Consider the reaction CO(g) + 3 H2 (g) ⇌ CH4 (g) + H2O(g) Kc = 7.7×10–23 at 25 °C. 1.50 mol CH4 and...
Questions

Mathematics, 22.08.2019 12:50


Biology, 22.08.2019 12:50




Biology, 22.08.2019 12:50


Biology, 22.08.2019 12:50

Mathematics, 22.08.2019 12:50


Mathematics, 22.08.2019 12:50

Geography, 22.08.2019 12:50

Mathematics, 22.08.2019 12:50

Biology, 22.08.2019 12:50

English, 22.08.2019 12:50



History, 22.08.2019 12:50

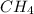 ,
, 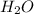 ,
, 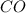 and
and 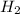 at equilibrium is, 0 mol, 1 mol, 1.5 mol and 4.5 mol respectively.
at equilibrium is, 0 mol, 1 mol, 1.5 mol and 4.5 mol respectively.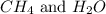
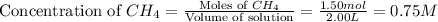
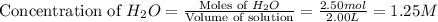
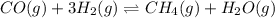
![K_c=\frac{[CH_4][H_2O]}{[CO][H_2]^3}](/tpl/images/0557/4993/fca95.png)