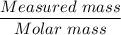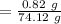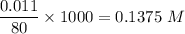Chemistry, 21.03.2020 08:05 lifeofabe214
A solution is composed of 1.15 mL of diethyl ether (C4H10O, density=0.7134 g/mL) dissolved in 78.85 mL of dichloromethane (CH2Cl2, density=1.3266 g/mL). What is the molarity of the solution?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2


Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
A solution is composed of 1.15 mL of diethyl ether (C4H10O, density=0.7134 g/mL) dissolved in 78.85...
Questions




English, 15.12.2020 18:10

Mathematics, 15.12.2020 18:10




History, 15.12.2020 18:10


Mathematics, 15.12.2020 18:10


Mathematics, 15.12.2020 18:10

Mathematics, 15.12.2020 18:10


History, 15.12.2020 18:10



English, 15.12.2020 18:10

Mathematics, 15.12.2020 18:10