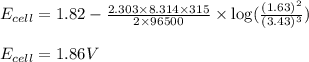A galvanic cell at a temperature of 42 degrees Celcius is powered by the following redox reaction:
3CU2+(aq) + 2Al(s) > 3Cu(s) + 2Al3+(aq)
Suppose the cell is prepared with 3.43 M Cu2+n one half-cell and 1.63 M Al3+in the other.
A) Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
A galvanic cell at a temperature of 42 degrees Celcius is powered by the following redox reaction:
Questions

Mathematics, 16.11.2019 06:31

Computers and Technology, 16.11.2019 06:31

Mathematics, 16.11.2019 06:31


Mathematics, 16.11.2019 06:31


Mathematics, 16.11.2019 06:31

Physics, 16.11.2019 06:31

History, 16.11.2019 06:31











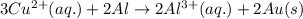
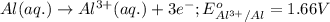 ( × 2)
( × 2)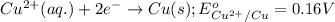 ( × 3)
( × 3)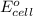 of the reaction, we use the equation:
of the reaction, we use the equation: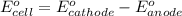
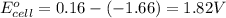
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Al^{3+}]^2}{[Cu^{2+}]^3}](/tpl/images/0557/5138/3aff8.png)
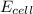 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![42^oC=[42+273]K=315K](/tpl/images/0557/5138/563a7.png)
![[Al^{3+}]=1.63M](/tpl/images/0557/5138/1e5e1.png)
![[Cu^{2+}]=3.43M](/tpl/images/0557/5138/455ea.png)