Chemistry, 21.03.2020 08:35 alexusjones6042
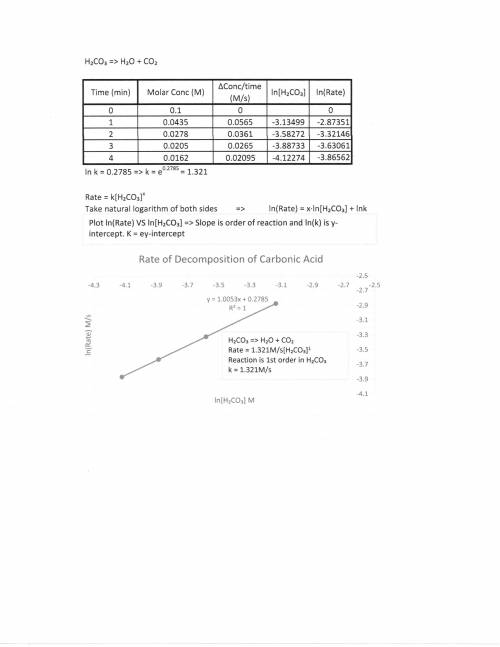
A chemistry graduate student is studying the rate of this reaction: H2CO3(aq) →H2O(aq)+CO2(aq)
He fills a reaction vessel with H2CO3 and measures its concentration as the reaction proceeds:
time (minutes) H2CO3
0 0.100M
1.0 0.0435M
2.0 0.0278M
3.0 0.0205M
4.0 0.0162M
write the reate law for this reaction: k ?
calculate the value of the rate constant k. Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbols.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3


Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
A chemistry graduate student is studying the rate of this reaction: H2CO3(aq) →H2O(aq)+CO2(aq)
Questions

Mathematics, 05.05.2020 15:25

Social Studies, 05.05.2020 15:25




Mathematics, 05.05.2020 15:25


Mathematics, 05.05.2020 15:25

Mathematics, 05.05.2020 15:25



English, 05.05.2020 15:25


Biology, 05.05.2020 15:25

Mathematics, 05.05.2020 15:25



English, 05.05.2020 15:25

Geography, 05.05.2020 15:25