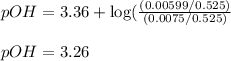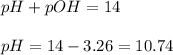Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1


Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1
You know the right answer?
Calculate the pH of a buffer that is prepared by mixing 25.0 mL of 0.300 M methylamine (CH3NH2) and...
Questions



Chemistry, 16.12.2020 20:30


Spanish, 16.12.2020 20:30




History, 16.12.2020 20:30

Biology, 16.12.2020 20:30






History, 16.12.2020 20:30

History, 16.12.2020 20:30

Mathematics, 16.12.2020 20:30


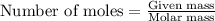
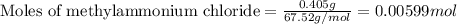
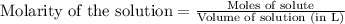

![pOH=pK_b+\log(\frac{[salt]}{[base]})](/tpl/images/0557/6860/db86b.png)
![pOH=pK_b+\log(\frac{[CH_3NH_3^+Cl^-]}{[CH_3NH_2]})](/tpl/images/0557/6860/9a761.png)
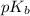 = negative logarithm of base dissociation constant of methylamine = 3.36
= negative logarithm of base dissociation constant of methylamine = 3.36![[CH_3NH_2]=\frac{0.0075}{0.525}](/tpl/images/0557/6860/37e87.png)
![[CH_3NH_3^+Cl^-]=\frac{0.00599}{0.525}](/tpl/images/0557/6860/55682.png)