
Chemistry, 21.03.2020 21:34 emilyturchon
C12H22011+1202-->12CO2+11H20
if 49.8 L of O2 are consumed, how many moles of sucrose are required?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

You know the right answer?
C12H22011+1202-->12CO2+11H20
if 49.8 L of O2 are consumed, how many moles of sucrose are re...
if 49.8 L of O2 are consumed, how many moles of sucrose are re...
Questions

Mathematics, 03.12.2020 18:40

Mathematics, 03.12.2020 18:40

Mathematics, 03.12.2020 18:40

English, 03.12.2020 18:40


English, 03.12.2020 18:40

Mathematics, 03.12.2020 18:40

Mathematics, 03.12.2020 18:40


Mathematics, 03.12.2020 18:40




English, 03.12.2020 18:40



Business, 03.12.2020 18:40



 at STP
at STP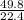 = 2.22moles
= 2.22moles 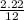 = 0.185moles
= 0.185moles

